Solar energy has the greatest potential to replace fossil fuels among all carbon-free energy sources. Given that electricity only accounts for 30% of global energy consumption, breakthroughs in renewable energy storage are needed to fulfil the rest of global energy requirement. The chemical bonds found in fuels provide one of the densest ways to store energy. For example, energy density of hydrogen fuel is more than 100 times higher than that of a best Li-ion battery. The ability to convert intermittent solar energy into storable chemical fuels is critical to the creation of a supply chain for renewable energy exports.
Solar Water Splitting
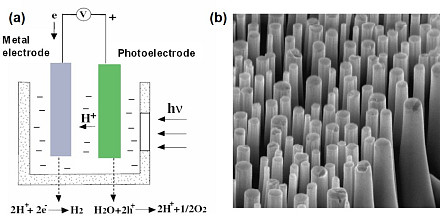
Hydrogen generation via photoelectrochemical (PEC) method is believed to be inexpensive in which the key components are the semiconductor electrodes. The highest solar-to-hydrogen conversion efficiencies have been achieved using III-V semiconductor materials. Many III-V semiconductors possess a range of desirable properties, including direct band gaps, high control of doping levels and formation of tunnel junctions, and excellent optoelectronic properties. However, practical application of III-V compound semiconductors in solar water splitting has been limited primarily by the high materials costs and short operational lifetimes.
Carbon Dioxide (CO2) Reduction
World’s heavy dependence on fossil fuels results in excessive emission of atmospheric CO2 caused by the combustion of fossil fuels. Sunlight-assisted CO2 reduction into higher energy chemicals, such as carbon monoxide, formic acid, methanol or methane provides a promising approach to alleviate both global warming and energy crisis. However, converting CO2 to useful chemicals in a selective and efficient manner remains a major research challenge. The PEC reduction process, which can integrate the benefits of both sunlight harvesting and selective catalysis, can provide an effective solution for CO2 reduction with high efficiency and excellent selectivity.
In these projects, students will get opportunities to work on the following subtopics.
Materials Science, Nanotechnology, Physical Chemistry, Semiconductor Physics