If humanity is to avoid catastrophic levels of global warming - i.e. stay under 2o C - we will need not only to slash emissions through renewable energy, but also remove hundreds of billions of tons of carbon dioxide (CO2) already in the atmosphere, and find locations to store this carbon. Forests can play a major role in such 'negative emissions' but will only achieve a fraction of the desired storage - the earth needs negative technologies such as direct air capture, coupled to underground storage.
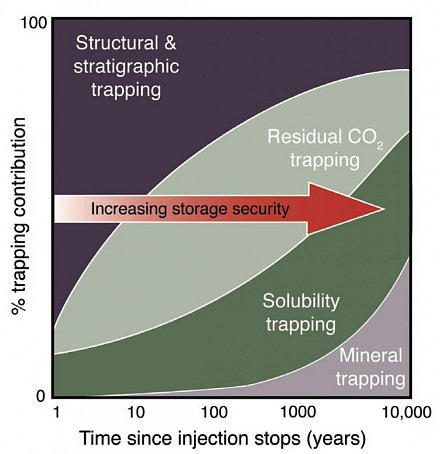
Subterranean carbon storage works via multiple mechanisms, as in the attached figure. It is a complex and interesting process governed by a balance between the tendency of less-dense CO2 plume to rise under buoyancy and the tendency of more-dense dissolved CO2 to sink under convective mixing. The buoyant CO2 plume (in a supercritical state due to the elevated pressures and temperatures) needs to be delayed in its upwards migration by the geology of the aquifer and by micro-scale capillary trapping in pores. If delayed for long enough the the CO2 will dissolve, form carbonic acid, and sink to locations where it will eventually form carbonate minerals.
To capture the dynamics of carbon storage involves four physical processes: buoyancy of bubbles, dissolution at interfaces, diffusion through water and downwards convective mixing. No scientific studies to date have comprehensively integrated these elements. This project aims to fill that gap: to conduct experiments and build models integrating these four processes, and use these models to identify conditions -- and therefore locations -- that are most favourable for CO2 storage.
Thermodynamics, from Physics or Chemistry courses.