The Space Plasma Power and Propulsion (SP3) group has been at the forefront of plasma physics and materials processing for more than two decades. SP3 has played a significant role in the semiconductor industry in developing new high-density plasma sources and their application to innovative thin film deposition. Currently, SP3 are bringing the 1970s microelectronic revolution to the hydrogen economy sector.

Since 2004 SP3 has been developing a new generation of proton exchange membrane (PEM) fuel cells. We are applying our expertise in plasma physics, plasma chemistry and materials processing to the manufacturing of efficient PEM fuel cells based around plasma processing techniques. In comparison to typical wet chemical processing of materials plasma processes are clean, dry and efficient. Several plasma processing techniques are being utilized to make fuel cells a more viable alternative energy source for the future. In fuel cells platinum plays a crucial role, but makes fuel cells very expensive. It has been shown that the amount of precious metals used for the catalyst (such as platinum) in PEM fuel cells can be significantly reduced using plasma-sputter deposition while at the same time increasing the fuel cell efficiency. Furthermore, we are implementing a plasma discharge to synthesize the plasma polymer membranes that are thin, dense and highly cross-linked.

Figure 1. A helicon plasma used for materials processing
The properties of thin films deposited from a plasma are critically dependent on the flux, energy and charge state of the particles striking the growing surface (1). Plasma sputter deposition of the platinum catalyst onto porous films of carbon has shown great promise: aggregates of platinum are detected in the film with a density profile which decreases away from the surface exposed to the plasma. The small size of the nano-aggregates allows very effective catalytic action necessary for efficient fuel cell operation (2-6). The energy deposited by the plasma can also be used to functionalise commercial Nafion proton exchange membranes (7) suggesting the necessity of controlling the various plasma parameters for alternate scenarios such as the plasma deposition of PEM (8) and the continuous plasma deposition of the MEA (Membrane Electrode Assembly) (9). Plasmas improve deposition quality, decrease process time, and open new paths for rapid commercialization.


Figure 2.The plasma sputter deposition reactor, Piglet, and plasma deposition of the catalyst
Although a thick (about 30um) active ink containing porous carbon, platinum, teflon and some nafion, is chemically made and 'painted' onto the electrode surface, the catalytic activity occurs mostly at the interface between the membrane and the electrode over a thickness of a few microns only. With plasma technology, it is possible to reduce the thickness of the catalytic layer (hence keeping the platinum use to a minimum), to increase the effective surface area for maximising catalytic activity for a fixed platinum loading, and to investigate the use of platinum gradients in the catalytic layer. Gradients can not be easily obtained when using chemical fabrication methods. An initial plasma process elaborated in the 'CATAPULP' reactor (2-6) consists in an argon plasma sputtering a platinum target biased negatively so that the platinum atoms can subsequently be redeposited on the gas diffusion layer in the form of nano-aggregates to enhance the catalytic activity and minimize the amount of catalyst used.
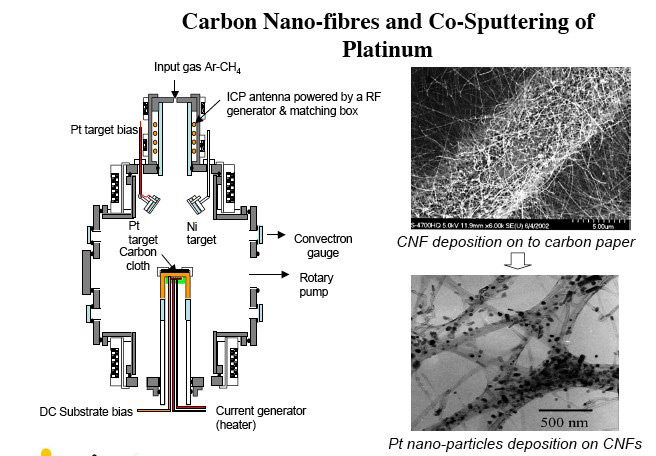
It is of interest to set up a plasma process where both the carbon and the platinum can be deposited with predetermined profiles. This can be achieved in 'CATAPULP' by adding a second carbon target in addition to the platinum target and by sputtering both targets with an argon plasma, where the sputtering rate control is obtained using various biases (9). Another method consists in using carbon-containing plasmas such as methane or acetylene and this is investigated in our new 'SOUTHERN CROSS' plasma reactor. The operating range of parameters is further increased by adding a temperature control of the electrode during the plasma process with the aim of further increasing the catalytic efficiency by depositing nano-aggregates of platinum on nano-structures of high conductivity graphitic carbon while maintaining a sufficient thin film porosity. The porosity has to be sufficiently high in order to prevent gas choking in high current fuel cell operation, as shown by simulations of low temperature fuel cell electro-chemical performances.
Commercially available Nafion is an ion exchange membrane which is often used in PEMFCs. It consists of fluorocarbon chains terminated by sulfonic groups (SO3-), hence combining hydrophilic (in the bulk) and hydrophobic (at the surface) properties necessary for a good proton conductivity from the anode (H2 side) to the cathode (O2 side) and good drainage of the water formed from catalysis at the cathode. In operation the membrane needs to be hydrated. Recent studies have shown how plasmas can be used for the surface treatment of Nafion (5) or for the plasma assisted deposition of 'Nafion-like' polymers (3,4,7). It has been shown that bombarding the surface of Nation with 1 keV Ar ion doses of 10^15 to 10^17cm^-2 changes the surface roughness and hydrophobicity of the membrane (with increased fuel cell performance) without altering the proton conductivity. We have studied (3,4) the analysis of the change in hydrophobicity of Nafion samples treated in a low energy plasma diffusing from a radiofrequency helicon source in two distinct reactors 'CHI KUNG' and 'PIGLET'. Although our results are strikingly different to those obtained by Cho et al, we have shown that the treated surface exhibits a decrease in hydrophobicity with an increased energy dose. This has been modelled using low pressure plasma theory. The energy dose provided by the ion bombardment during the plasma treatment is responsible for this change while the energy dose from UV light has little effect for the present energy range of a few watts per square centimeter. Further experiments will focus on fuel cell performances using our new test bench.

Figure 3. Chemical PEM Fuel cell : structure
Mahdjoub et al (9) have recently shown that thin films of plasma-polymerised proton conductive membranes could be deposited by plasma polymerization of trifluoromethane sulfonic acid (CF3SO3H), 1,3-butadiene and styrene mixtures in glow or afterglow argon discharges. Their results suggest that the type of discharge has an important impact on the micro-structure of the plasma deposited film and on the resulting proton conductivity. Currently, we are in the process of setting up a plasma enhanced chemical vapour deposition (PECVD) system to synthesize the plasma polymer membranes. These polymers have been shown to be thin, dense and highly cross-linked. They have a very good chemical and thermal stability and have a low permeability to organic liquids such as methanol. However, the proton conductivity is lower than that of Nafion.

Piglet, an SP3 helicon sputter deposition reactor

Piglet, in the lab
The Southern Cross plasma reactor
(1) M.A. Lieberman and A.J. Lichtenberg, Principles of Plasma Discharges and Materials Processing, 2nd ed., Wiley, New York (2005).
(2) P. Brault, S. Roualdes, A. Caillard, A.L. Thomann, J. Mathias, J. Durand, C. Coutanceau, J.M. Leger, C. Charles, and R. Boswell "Solid Polymer Fuel Cell synthesis by low pressure plasmas: A short review" Eur. Phys. J. Appl. Phys. 34, 151-156 (2006)
(3) C. Charles, D. Ramdutt, P. Brault, A. Caillard, D. Bulla, R.W. Boswell and A. Dicks "Low energy plasma treatment of a proton exchange membrane used for low temperature fuel cells". Accepted in Plasma Physics and Controlled Fusion (2006).
(4) C. Charles, A. Caillard, P. Brault, R.W. Boswell, D. Ramdutt, C. Corr, H. Rabat and A. Dicks "Laboratory Plasmas Applied to the Hydrogen Economy". Proceedings of the Alternate Transport Energies Conference (2006).
(5) P. Brault, A. Caillard, A.L. Thomann, J. Mathias, C. Charles, R. Boswell, S. Escribano, J. Durand, S. Roualdes, T. Sauvage "Plasma sputtering deposition of platinum into porous fuel cell electrodes" J. Phys. D: Appl. Phys. 37, 3419-3423 (2004)
(6) A. Caillard, P. Brault, J. Mathias, C. Charles, and R.W. Boswell "Deposition and diffusion of platinum nanoparticles in porous carbon assisted by plasma sputtering" Surf. Coat. Technol. 200, 391-394 (2005)
(7) S.A. Cho, E.A. Cho, I.H. Oh, H.J. Kim, H.Y. Ha, S.A. Hong, J.B. Ju "Surface modified Nafion membrane by ion beam bombardment for fuel cell applications" Journal of Power Sources 155, 286-290 (2006)
(8) H. Mahdjoub, S. Roualdes, P. Sistat, N. Pradeilles, J. Durand, and G. Pourcelly "Plasma-Polymerised Proton Conductive Membranes for a miniaturised PEMFC" Fuel Cells 5, 277 (2005)
(9) H. Rabat, A. Caillard, P. Brault, C. Charles, R.W. Boswell Private communication, May 2006 Return to Fuel Cell Background Information Page Document Actions