Carbon is a very versatile atom due to its valence of either +4 or -4, capable of forming many different monoatomic structures with unique mechanical as well as electronical properties. Under high pressure conditions, diamond is the stable form of pure carbon, characterised by a 3D framework of sp3 hybridised covalent bonds which leads to the extreme hardness and durability of diamond. Simulations suggest, that there might be more stable sp3 hybridised carbon phases: Over 522 individual stable or metastable structures have been predicted as of May 2017, some of them potentially exceeding the hardness of diamond. However, in experimental high pressure research only two other phases were reported apart from diamond, and both are quite controversial. My research project aims to explore the possibility of forming different carbon phases by utilising different carbon samples as precursor materials under non-hydrostatic stress fields.
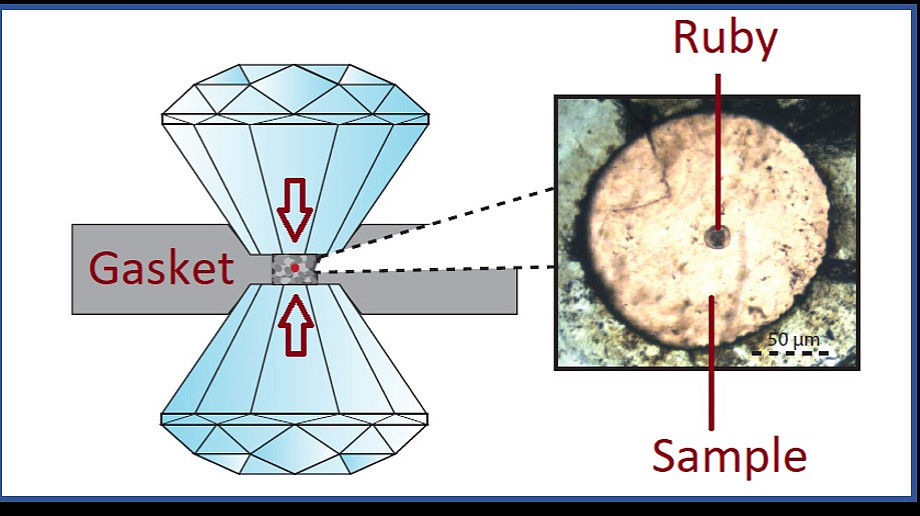
In my talk I will give an introduction to high pressure research utilising diamond anvil cell technology with in situ Raman spectroscopy and in situ X-ray diffraction. In our ANU lab we can achieve pressures of up to 200 GPa in diamond anvil cells, which is about half the pressure compared to the pressure at the centre of the earth’s core, or in other words: 5000 times more than the pressure at the wreck of the Titanic. Furthermore, I will present results from my first two PhD research years focusing on pressurising two types of molecular carbon materials. Firstly, I will elaborate on phase transformations in diamondoid molecular crystals. Diamondoids are hydrocarbons composed of a fully sp3 bonded diamond-like carbon cage. Remarkably, these molecules stay fully inert after compressions to over 50 GPa, making them interesting candidates for cushioning applications. Secondly, I will present an amorphous diamond-like material intertwined by nanocrystalline diamond bands formed by collapsing C60 fullerene cages at pressures of around 50 GPa at ambient temperatures.
Room:
Conference Room (4.03)